Drastic effect of the peptide sequence on the copper-binding properties of tripeptides and the electrochemical behaviour of their copper(II) complexes
Silvia Mena, Andrea Mirats, Ana B. Caballero, Gonzalo Guirado, Leoní A. Barrios, Simon J. Teat, Luis Rodriguez-Santiago, Mariona Sodupe and Patrick Gamez
Chem. Eur. J. 2018, 24, 5153-5162
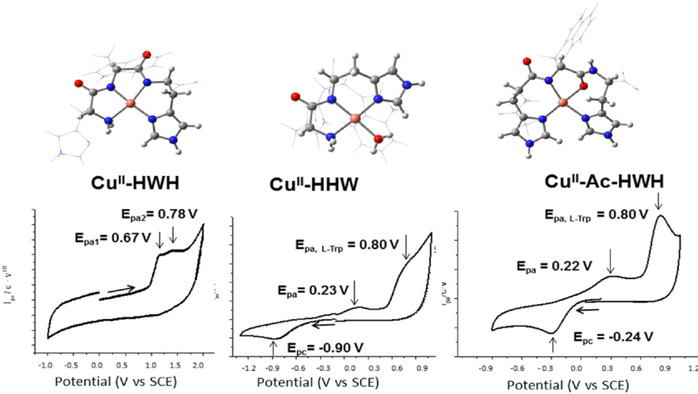
The binding and electrochemical properties of the complexes CuII‐HAH, CuII‐HWH, CuII‐Ac‐HWH, CuII‐HHW, and CuII‐WHH have been studied by using NMR and UV/Vis spectroscopies, CV, and density functional calculations. The results obtained highlight the importance of the peptidic sequence on the coordination properties and, consequently, on the redox properties of their CuII complexes. For CuII‐HAH and CuII‐HWH, no cathodic processes are observed up to −1.2 V; that is, the complexes exhibit very high stability towards copper reduction. This behaviour is associated with the formation of very stable square‐planar (5,5,6)‐membered chelate rings (ATCUN motif), which enclose two deprotonated amides. In contrast, for non‐ATCUN CuII‐Ac‐HWH, CuII‐HHW complexes, simulations seem to indicate that only one deprotonated amide is enclosed in the coordination sphere. In these cases, the main electrochemical feature is a reductive irreversible one electron‐transfer process from CuII to CuI, accompanied with structural changes of the metal coordination sphere and reprotonation of the amide. Finally, for CuII‐WHH, two major species have been detected: one at low pH (<5), with no deprotonated amides, and another one at high pH (>10) with an ATCUN motif, both species coexisting at intermediate pH. The present study shows that the use of CV, using glassy carbon as a working electrode, is an ideal and rapid tool for the determination of the redox properties of CuII metallopeptides.